In vitro diagnostics quality controls have taken on a crucial role in modern healthcare due to the better accuracy of diagnosis offered by their use.
In 2018, the global In Vitro Diagnostics Quality Control market size was xx million US$ and it is expected to reach xx million US$ by the end of 2025, with a CAGR of xx% during 2019-2025.
This report focuses on the global In Vitro Diagnostics Quality Control status, future forecast, growth opportunity, key market and key players. The study objectives are to present the In Vitro Diagnostics Quality Control development in United States, Europe and China.
The key players covered in this study
Abbott Laboratories
Bio-Rad
Helena Laboratories
Ortho Clinical Diagnostics
Randox Laboratories
Roche
Seracare
Siemens Healthineers
Sun Diagnostics
Thermo Fisher Scientific
Sysmex
Quantimetrix
Market segment by Type, the product can be split into
Whole Blood Based Controls
Serum/Plasma Based Controls
Urine Based Controls
Data Management Solutions
Quality Assurance Services
Market segment by Application, split into
Hospitals
Clinical Laboratories
Research and Academic Institutes
Market segment by Regions/Countries, this report covers
United States
Europe
China
Japan
Southeast Asia
India
Central & South America
The study objectives of this report are:
To analyze global In Vitro Diagnostics Quality Control status, future forecast, growth opportunity, key market and key players.
To present the In Vitro Diagnostics Quality Control development in United States, Europe and China.
To strategically profile the key players and comprehensively analyze their development plan and strategies.
To define, describe and forecast the market by product type, market and key regions.
In this study, the years considered to estimate the market size of In Vitro Diagnostics Quality Control are as follows:
History Year: 2014-2018
Base Year: 2018
Estimated Year: 2019
Forecast Year 2019 to 2025
For the data information by region, company, type and application, 2018 is considered as the base year. Whenever data information was unavailable for the base year, the prior year has been considered.
Table of Contents
1 Report Overview
1.1 Study Scope
1.2 Key Market Segments
1.3 Players Covered
1.4 Market Analysis by Type
1.4.1 Global In Vitro Diagnostics Quality Control Market Size Growth Rate by Type (2014-2025)
1.4.2 Whole Blood Based Controls
1.4.3 Serum/Plasma Based Controls
1.4.4 Urine Based Controls
1.4.5 Data Management Solutions
1.4.6 Quality Assurance Services
1.5 Market by Application
1.5.1 Global In Vitro Diagnostics Quality Control Market Share by Application (2014-2025)
1.5.2 Hospitals
1.5.3 Clinical Laboratories
1.5.4 Research and Academic Institutes
1.6 Study Objectives
1.7 Years Considered
2 Global Growth Trends
2.1 In Vitro Diagnostics Quality Control Market Size
2.2 In Vitro Diagnostics Quality Control Growth Trends by Regions
2.2.1 In Vitro Diagnostics Quality Control Market Size by Regions (2014-2025)
2.2.2 In Vitro Diagnostics Quality Control Market Share by Regions (2014-2019)
2.3 Industry Trends
2.3.1 Market Top Trends
2.3.2 Market Drivers
2.3.3 Market Opportunities
3 Market Share by Key Players
3.1 In Vitro Diagnostics Quality Control Market Size by Manufacturers
3.1.1 Global In Vitro Diagnostics Quality Control Revenue by Manufacturers (2014-2019)
3.1.2 Global In Vitro Diagnostics Quality Control Revenue Market Share by Manufacturers (2014-2019)
3.1.3 Global In Vitro Diagnostics Quality Control Market Concentration Ratio (CR5 and HHI)
3.2 In Vitro Diagnostics Quality Control Key Players Head office and Area Served
3.3 Key Players In Vitro Diagnostics Quality Control Product/Solution/Service
3.4 Date of Enter into In Vitro Diagnostics Quality Control Market
3.5 Mergers & Acquisitions, Expansion Plans
4 Breakdown Data by Type and Application
4.1 Global In Vitro Diagnostics Quality Control Market Size by Type (2014-2019)
4.2 Global In Vitro Diagnostics Quality Control Market Size by Application (2014-2019)
5 United States
5.1 United States In Vitro Diagnostics Quality Control Market Size (2014-2019)
5.2 In Vitro Diagnostics Quality Control Key Players in United States
5.3 United States In Vitro Diagnostics Quality Control Market Size by Type
5.4 United States In Vitro Diagnostics Quality Control Market Size by Application
6 Europe
6.1 Europe In Vitro Diagnostics Quality Control Market Size (2014-2019)
6.2 In Vitro Diagnostics Quality Control Key Players in Europe
6.3 Europe In Vitro Diagnostics Quality Control Market Size by Type
6.4 Europe In Vitro Diagnostics Quality Control Market Size by Application
7 China
7.1 China In Vitro Diagnostics Quality Control Market Size (2014-2019)
7.2 In Vitro Diagnostics Quality Control Key Players in China
7.3 China In Vitro Diagnostics Quality Control Market Size by Type
7.4 China In Vitro Diagnostics Quality Control Market Size by Application
8 Japan
8.1 Japan In Vitro Diagnostics Quality Control Market Size (2014-2019)
8.2 In Vitro Diagnostics Quality Control Key Players in Japan
8.3 Japan In Vitro Diagnostics Quality Control Market Size by Type
8.4 Japan In Vitro Diagnostics Quality Control Market Size by Application
9 Southeast Asia
9.1 Southeast Asia In Vitro Diagnostics Quality Control Market Size (2014-2019)
9.2 In Vitro Diagnostics Quality Control Key Players in Southeast Asia
9.3 Southeast Asia In Vitro Diagnostics Quality Control Market Size by Type
9.4 Southeast Asia In Vitro Diagnostics Quality Control Market Size by Application
10 India
10.1 India In Vitro Diagnostics Quality Control Market Size (2014-2019)
10.2 In Vitro Diagnostics Quality Control Key Players in India
10.3 India In Vitro Diagnostics Quality Control Market Size by Type
10.4 India In Vitro Diagnostics Quality Control Market Size by Application
11 Central & South America
11.1 Central & South America In Vitro Diagnostics Quality Control Market Size (2014-2019)
11.2 In Vitro Diagnostics Quality Control Key Players in Central & South America
11.3 Central & South America In Vitro Diagnostics Quality Control Market Size by Type
11.4 Central & South America In Vitro Diagnostics Quality Control Market Size by Application
12 International Players Profiles
12.1 Abbott Laboratories
12.1.1 Abbott Laboratories Company Details
12.1.2 Company Description and Business Overview
12.1.3 In Vitro Diagnostics Quality Control Introduction
12.1.4 Abbott Laboratories Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.1.5 Abbott Laboratories Recent Development
12.2 Bio-Rad
12.2.1 Bio-Rad Company Details
12.2.2 Company Description and Business Overview
12.2.3 In Vitro Diagnostics Quality Control Introduction
12.2.4 Bio-Rad Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.2.5 Bio-Rad Recent Development
12.3 Helena Laboratories
12.3.1 Helena Laboratories Company Details
12.3.2 Company Description and Business Overview
12.3.3 In Vitro Diagnostics Quality Control Introduction
12.3.4 Helena Laboratories Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.3.5 Helena Laboratories Recent Development
12.4 Ortho Clinical Diagnostics
12.4.1 Ortho Clinical Diagnostics Company Details
12.4.2 Company Description and Business Overview
12.4.3 In Vitro Diagnostics Quality Control Introduction
12.4.4 Ortho Clinical Diagnostics Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.4.5 Ortho Clinical Diagnostics Recent Development
12.5 Randox Laboratories
12.5.1 Randox Laboratories Company Details
12.5.2 Company Description and Business Overview
12.5.3 In Vitro Diagnostics Quality Control Introduction
12.5.4 Randox Laboratories Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.5.5 Randox Laboratories Recent Development
12.6 Roche
12.6.1 Roche Company Details
12.6.2 Company Description and Business Overview
12.6.3 In Vitro Diagnostics Quality Control Introduction
12.6.4 Roche Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.6.5 Roche Recent Development
12.7 Seracare
12.7.1 Seracare Company Details
12.7.2 Company Description and Business Overview
12.7.3 In Vitro Diagnostics Quality Control Introduction
12.7.4 Seracare Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.7.5 Seracare Recent Development
12.8 Siemens Healthineers
12.8.1 Siemens Healthineers Company Details
12.8.2 Company Description and Business Overview
12.8.3 In Vitro Diagnostics Quality Control Introduction
12.8.4 Siemens Healthineers Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.8.5 Siemens Healthineers Recent Development
12.9 Sun Diagnostics
12.9.1 Sun Diagnostics Company Details
12.9.2 Company Description and Business Overview
12.9.3 In Vitro Diagnostics Quality Control Introduction
12.9.4 Sun Diagnostics Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.9.5 Sun Diagnostics Recent Development
12.10 Thermo Fisher Scientific
12.10.1 Thermo Fisher Scientific Company Details
12.10.2 Company Description and Business Overview
12.10.3 In Vitro Diagnostics Quality Control Introduction
12.10.4 Thermo Fisher Scientific Revenue in In Vitro Diagnostics Quality Control Business (2014-2019)
12.10.5 Thermo Fisher Scientific Recent Development
12.11 Sysmex
12.12 Quantimetrix
13 Market Forecast 2019-2025
13.1 Market Size Forecast by Regions
13.2 United States
13.3 Europe
13.4 China
13.5 Japan
13.6 Southeast Asia
13.7 India
13.8 Central & South America
13.9 Market Size Forecast by Product (2019-2025)
13.10 Market Size Forecast by Application (2019-2025)
14 Analyst's Viewpoints/Conclusions
15 Appendix
15.1 Research Methodology
15.1.1 Methodology/Research Approach
15.1.1.1 Research Programs/Design
15.1.1.2 Market Size Estimation
12.1.1.3 Market Breakdown and Data Triangulation
15.1.2 Data Source
15.1.2.1 Secondary Sources
15.1.2.2 Primary Sources
15.2 Disclaimer
15.3 Author Details
SDMR employs a three way data triangulation approach to arrive at market estimates. We use primary research, secondary research and data triangulation by top down and bottom up approach
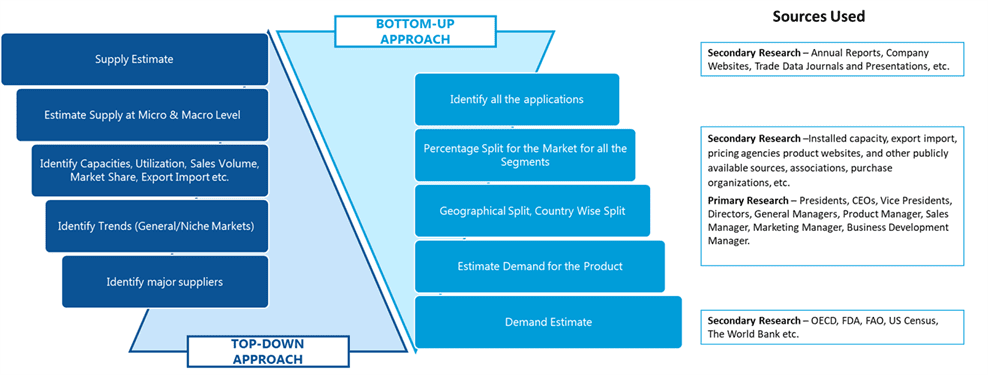
Secondary Research:
Our research methodology involves in-depth desk research using various secondary sources. Data is gathered from association/government publications/databases, company websites, press releases, annual reports/presentations/sec filings, technical papers, journals, research papers, magazines, conferences, tradeshows, and blogs.
Key Data Points through secondary research-
Macro-economic data points
Import Export data
Identification of major market trends across various applications
Primary understanding of the industry for both the regions
Competitors analysis for the production capacities, key production sites, competitive landscape
Key customers
Production Capacity
Pricing Scenario
Cost Margin Analysis
Key Data Points through primary research-
Major factors driving the market and its end application markets
Comparative analysis and customer analysis
Regional presence
Collaborations or tie-ups
Annual Production, and sales
Profit Margins
Average Selling Price
Data Triangulation:
Data triangulation is done using top down and bottom approaches. However, to develop accurate market sizing estimations, both the methodologies are used to accurately arrive at the market size. Insert Image

